About
PCORnet is a national resource that offers the kind of research ecosystem that has long been pursued: a fully integrated network where vast, highly representative health data, research expertise, and patient insights are built-in and accessible from the very start. The infrastructure of PCORnet is well established, meaning that PCORnet® Network Partners know how to maximize the value of these connections to deliver fast, trustworthy answers that advance public health.
Research
PCORnet is uniquely networked to offer the real-world evidence essential for answering the clinical questions that impact peoples' lives and support studies addressing major public health issues.
Data
With access to data from everyday healthcare encounters with more than 30 million people annually across the U.S., users of PCORnet have potential insights at their fingertips. These data are accessible via a distributed research network model of eight large PCORnet® Clinical Research Networks (CRNs) and facilitated by the Coordinating Center for PCORnet®.
PCORnet: An Introduction
Get an overview of PCORnet in this fact sheet or learn more in this overview presentation for industry stakeholders, academic audiences, and health services researchers
Knock on the PCORnet® Front Door to access PCORnet
PCORnet is well suited to support the conduct of:
- Real-world evidence studies
- Comparative clinical effectiveness research
- Population health research
- Pragmatic research
- Health systems research
- And more
Engagement
When patients and caregivers take on an active role in research, outcomes improve. That’s why every effort powered by PCORnet broadly embeds the voice of patients and those who care for them. Patients and caregivers serve on the PCORnet® Steering Committee and provide leadership across every stage of research.
Network
PCORnet® Network Partners not only source data, but also propel patient-centered research forward. PCORnet® CRNs include the nation’s leading clinical researchers whose collective knowledge and experiences empower and support users of the Network.

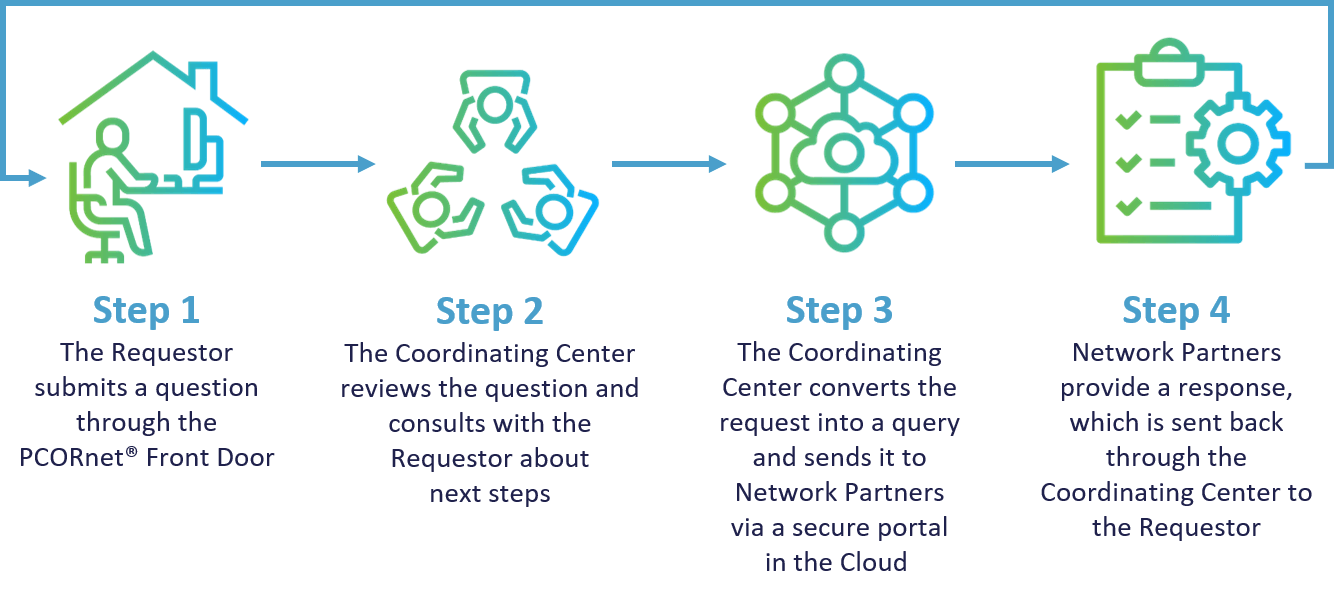
How It All Works
The PCORnet® Front Door is a central gateway point for anyone seeking to use PCORnet resources. Once users have reached out, the Coordinating Center for PCORnet® sets up a consultation to clarify the request and convert the question into a query to send out to the PCORnet® Network Partners for the best fit.