Engagement
Engaged communities drive better, faster research.
What do we mean by “communities”?
- Patients and caregivers
- Clinicians/Clinic Staff
- Insurers
- Policymakers
- Others

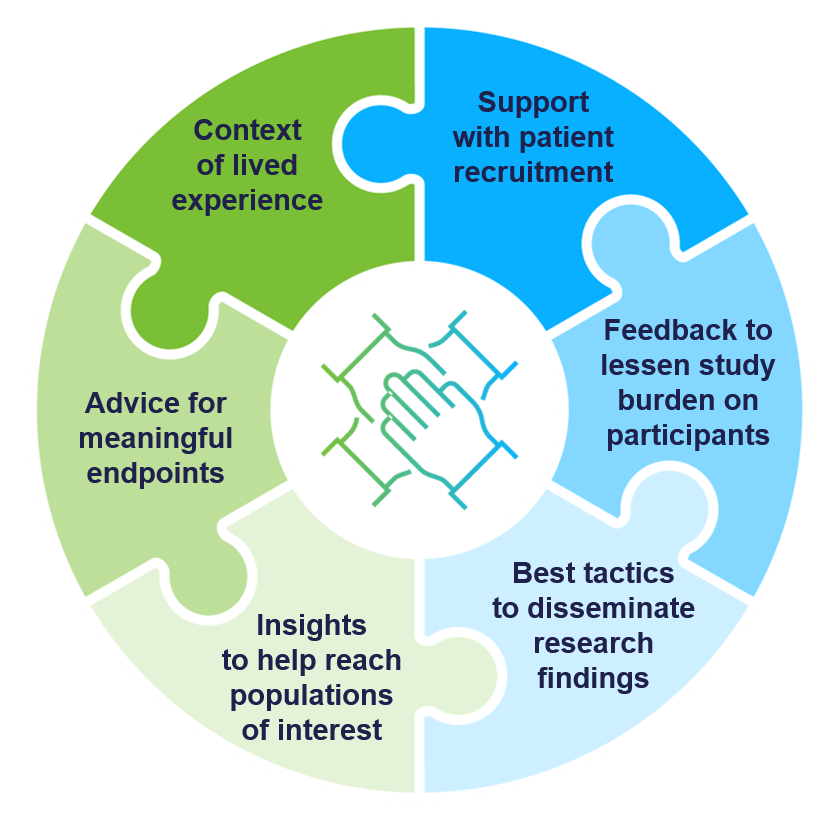
Engagement in PCORnet-enabled research is a two-way street.
Patients and caregivers have a seat at the table of every PCORnet® Study, engaging as coequal collaborators with health professionals.
From the Researcher Perspective

- Patients offer important context, directly informing understanding of a condition, its effects, and the burden of illness
- Patients can identify processes or procedures that research participants may find too burdensome, allowing researchers to amend and potentially boost study enrollment
- Patients ensure endpoints are meaningful, helping researchers deliver results that will improve the patient experience
- Patients can mobilize patient groups for participation in clinical trials
- Patients are essential partners in helping to disseminate results in a way that is clear to diverse communities
From the Patient Perspective

- Partnering in research empowers patients and caregivers to set the research agenda, advocating for prioritization of questions that matter to their community
- Patient partners can influence meaningful changes to study designs, giving these projects the best chance of success
- Patient partners report positive experiences in participating in and contributing to research that leads to improved clinical answers
- Patients drive adoption of actionable findings and meaningful changes in clinical care
Meet the PCORnet Patient Partners
Input from PCORnet patient partners makes research better—its implementation has effectively accelerated study enrollment rates, improved protocol design, inspired more relevant research aims, and strengthened dissemination of results. PCORnet patient partners serve on the PCORnet® Steering Committee.

Ava Zebrick
Patient Representative

Crispin Goytia
Patient Representative

Greg Merritt
Patient Representative

Melissa Bronson
Patient Representative

Nadine Zemon
Patient Representative

Neely Williams
Patient Representative

Shirley Stowe
Patient Representative
